Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men
`
`
- Nelly Pitteloud, MD1,
- Vamsi K. Mootha, MD2,
- Andrew A. Dwyer, BA1,
- Megan Hardin, BA1,
- Hang Lee, PHD3,
- Karl-Fredrik Eriksson, MD4,
- Devjit Tripathy, MD, DM4,
- Maria Yialamas, MD1,
- Leif Groop, MD, PHD4,
- Dariush Elahi, PHD5 and
- Frances J. Hayes, MB, BCH, BAO1
+ Author Affiliations
1Reproductive Endocrine Unit of the Department of Medicine, Massachusetts General Ho2spital, Boston, Massachusetts
2Broad Institute, Harvard University and Massachusetts Institute of Technology, Cambridge, Massachusetts
3Department of Biostatistics and General Clinical Research Center, Massachusetts General Hospital, Boston, Massachusetts
4Department of Endocrinology, Wallenberg Laboratory, University Hospital MAS, Lund University, Malmo, Sweden
5Department of Surgery, University of Massachusetts Medical Center, Worcester, Massachusetts
- Address correspondence and reprint requests to Frances J. Hayes, MD, Reproductive Endocrine Unit, BHX 511 Massachusetts General Hospital, 55 Fruit St., Boston, MA 02114. E-mail: fhayes@partners.org
Abstract
OBJECTIVE”” The goal of this study was to examine the relationship between serum testosterone levels and insulin sensitivity and mitochondrial function in men.
RESEARCH DESIGN AND METHODS””A total of 60 men (mean age 60.5 ± 1.2 years) had a detailed hormonal and metabolic evaluation. Insulin sensitivity was measured using a hyperinsulinemic-euglycemic clamp. Mitochondrial function was assessed by measuring maximal aerobic capacity (Vo2max) and expression of oxidative phosphorylation genes in skeletal muscle.
RESULTS””A total of 45% of subjects had normal glucose tolerance, 20% had impaired glucose tolerance, and 35% had type 2 diabetes. Testosterone levels were positively correlated with insulin sensitivity (r = 0.4, P < 0.005). Subjects with hypogonadal testosterone levels (n = 10) had a BMI >25 kg/m2 and a threefold higher prevalence of the metabolic syndrome than their eugonadal counterparts (n = 50); this relationship held true after adjusting for age and sex hormone-binding globulin but not BMI. Testosterone levels also correlated with Vo2max (r = 0.43, P < 0.05) and oxidative phosphorylation gene expression (r = 0.57, P < 0.0001).
CONCLUSIONS””These data indicate that low serum testosterone levels are associated with an adverse metabolic profile and suggest a novel unifying mechanism for the previously independent observations that low testosterone levels and impaired mitochondrial function promote insulin resistance in men.
`
`Insulin resistance has assumed increasing importance as a risk factor for cardiovascular disease coincident with the dramatic increase in the prevalence of obesity in the Western world. Recent studies using genetic analysis (1,2), functional imaging (3,4), and animal models of differential aerobic capacity (5) have shed light on the role of mitochondrial function in inducing the metabolic disturbances characteristic of insulin-resistant states. Using gene set enrichment analysis to look for changes in sets of genes compiled on the basis of function, Mootha et al. (1) showed decreased maximal aerobic capacity (Vo2max) and decreased expression of mitochondrial genes involved in oxidative phosphorylation (OXPHOS) in men of Northern European descent with impaired glucose tolerance (IGT) and type 2 diabetes. Using quantitative real-time PCR, Patti et al. (2) reported decreased OXPHOS gene expression in a Mexican-American population of type 2 diabetic subjects as well as insulin-resistant first-degree relatives of type 2 diabetic subjects with normal glucose tolerance (NGT). Magnetic resonance spectroscopy has shown that decreased mitochondrial oxidative and phosphorylation activity underlies the insulin resistance seen with aging (3) and in lean offspring of patients with type 2 diabetes (4). Recent data from rats bred to have low aerobic capacity also implicate impaired mitochondrial function in the development of the cardiovascular risk profile characteristic of the metabolic syndrome (5).
Little is known about the interaction between testosterone levels and insulin sensitivity in men, in contrast to the abundant literature on this relationship in women (6). Cross-sectional studies demonstrate an inverse relationship between testosterone and fasting insulin levels in men independent of age, obesity, and body fat distribution (7–11). A link between testosterone deficiency and diabetes has also been suggested with the demonstration that men with type 2 diabetes have lower testosterone levels than weight-matched nondiabetic control subjects (12,13). In addition, six large prospective studies have shown that low testosterone levels predict development of type 2 diabetes in men (14–19). Two studies demonstrate a positive relationship between total testosterone levels and insulin sensitivity in normal (20) and diabetic men (21). In contrast, data on the relationship between free testosterone levels and insulin sensitivity are conflicting, with two studies showing no correlation (21,22), whereas a third study demonstrates a weak positive relationship (20).
Given that low testosterone levels predict development of type 2 diabetes in men (14–19) and that aging is accompanied by insulin resistance and a decline in testosterone secretion (23–25), we hypothesized that testosterone is an important modulator of insulin sensitivity and mitochondrial function in men. Thus, the aims of this study were to 1) examine the relationship between serum testosterone levels and insulin sensitivity in men across a wide spectrum of insulin sensitivity, 2) dissect the role of obesity and alterations in sex hormone-binding globulin (SHBG) levels in mediating this relationship, and 3) determine if there is an association between serum testosterone levels and mitochondrial function reflected by Vo2max and OXPHOS gene expression in skeletal muscle.
RESEARCH DESIGN AND METHODS
A total of 60 men aged 39-69 years (mean 60.5 ± 1.2) were enrolled in this study. Subjects with a history of testicular disorders or who were taking medications known to interfere with testosterone secretion/action or glucose homeostasis were excluded from the study. Healthy normal men, obese men, and men with newly diagnosed type 2 diabetes on no hypoglycemic agents were enrolled to cover the full spectrum of insulin sensitivity. A total of 42 Caucasian men, who were participants in the Malmo Prevention Study (26), were studied in Sweden. Some of the metabolic characteristics of this Swedish cohort have already been reported (1). There were 18 subjects enrolled in the U.S., of whom 16 were Caucasian and 2 were African-American. The study was approved by the Ethics Committee at Lund University and the Human Research Committee of Massachusetts General Hospital. All subjects provided written informed consent before the initiation of any study procedures.
Anthropometric measures
Height and weight were measured by standard procedures to calculate BMI as weight (kg) divided by height squared (m2). Waist-to-hip ratio (WHR) was calculated by measuring waist circumference at the level of the umbilicus and hip circumference at the level of the greatest hip girth. In the 42 Swedish subjects, percent body fat was measured using bioelectrical impedance analysis.
Oral glucose tolerance test
A standardized 2-h oral glucose tolerance test using 75 g glucose was performed on the basis of which subjects were classified as having NGT, IGT, or type 2 diabetes using 1985 criteria from the World Health Organization (27).
Insulin sensitivity
Insulin sensitivity was determined by the hyperinsulinemic-euglycemic clamp technique (28). For 3 days before the study, all subjects consumed a weight-maintaining diet containing 300 g carbohydrate per day. The clamp studies were performed at 7:30 a.m. after a 12-h fast. An intravenous cannula was inserted into an antecubital vein for the infusion of insulin and glucose. A second catheter was inserted retrogradely into a hand vein for blood sampling; the hand was kept heated in a warming chamber to arterialize venous samples. Blood glucose was measured every 5 min for 120 min. Forty-two clamps were performed with an insulin infusion rate of 40 mU · m−2 · min−1 with use of [3-3H]glucose to correct for hepatic glucose production. There were 18 clamps performed with an insulin infusion rate of 80 mU · m−2 · min−1, a dose sufficient to suppress hepatic glucose production. An infusion of 20% glucose was initiated and titrated to maintain blood glucose at the fasting level. The glucose disposal rate was determined during the last 30 min of the 2-h clamp. Under steady-state conditions of euglycemia, the rate of exogenous glucose infusion, corrected for glucose space and hepatic glucose production, is equal to the rate of insulin-stimulated glucose disposal, expressed as the M value (mg · kg−1 · min−1), a measure of insulin sensitivity. Mean insulin levels during the clamp were 497 ± 15 pmol/l and 1,188 ± 75 pmol/l for insulin doses of 40 mU · m−2 · min−1 and 80 mU · m−2 · min−1, respectively. All statistical analyses using insulin sensitivity as a parameter were corrected for insulin levels during the clamp.
Maximal aerobic capacity
Vo2max was measured in the Swedish subjects using an incremental work-conducted upright exercise test with a bicycle ergometer (Monark Varberg, Sweden) combined with continuous analysis of expiratory gases and minute ventilation. Exercise was started at a workload varying between 30 and 100 W depending on the history of endurance training or exercise habits and increased by 20-50 W every 3 min, until a perceived exhaustion or a respiratory quotient of 1.0 was reached. Maximal aerobic capacity was defined as the Vo2 during the last 30 s of exercise and is expressed per lean body mass calculated by bioelectrical impedance analysis.
Percutaneous muscle biopsy
Percutaneous muscle biopsies (20-50 mg) were taken from the vastus lateralis muscle of the Swedish subjects. Biopsies were performed under local anesthesia (1% lidocaine) using a Bergström needle after the hyperinsulinemic-euglycemic clamp. Fiber-type composition and whole-muscle glycogen concentration were determined as previously described (29). Quantification and calculation of the fibers was performed using the COMFAS image analysis system (Scan Beam, Hadsun, Denmark).
Gene set enrichment analysis
Total RNA was isolated from the muscle biopsies, and the targets were hybridized to the Affymetrix HG-U133A chip. Marker analysis was first performed using GeneCluster as previously described (1). The software package SAM was used to identify genes on the microarrays with statistically significant changes in expression. The approach of gene set enrichment analysis was then used to analyze previously defined sets of functionally related genes and search for systematic expression differences among the genes in the set according to glucose tolerance.
Using this technique, a subset of genes involved in oxidative phosphorylation (OXPHOS-CR) was recently identified that is tightly coregulated across many tissues, is highly expressed in sites of insulin-mediated glucose disposal, and for which expression is decreased by ∼20% in skeletal muscle of men with IGT and type 2 diabetes (1). Of the 34 genes in the OXPHOS-CR set, the top-ranking gene (i.e., the gene with the largest expression difference between normal and diabetic muscle) was ubiquinol cytochrome c reductase-binding protein (UQCRB). In the present study, the data on OXPHOS-CR gene expression were analyzed to determine if there was an association with serum testosterone levels.
Biochemical analysis
Fasting blood samples collected before the start of the glucose clamp at −30, −20, and −10 min were pooled and used to determine serum levels of testosterone, estradiol (E2), SHBG, total and HDL cholesterol, triglycerides, and free fatty acids.
Immunoassays
Serum testosterone concentrations were measured using the DPC Coat-A-Count RIA kit, which has an intra- and interassay coefficient of variation (CV) of <10%. E2 was measured with an RIA using hexane ethylacetate extraction and LH-20 chromatography (Esoterix, Calabasas Hills, CA). The E2 assay has a sensitivity of 18 pmol/l and, based on a male serum pool, has an intra-assay CV of 4.9% and an interassay CV of 15%. Plasma glucose was measured with the glucose oxidase method (Beckman Instruments, Fullerton, CA). Insulin was measured by RIA using 125I-labeled human insulin and human insulin antiserum (Linco Research, St. Charles, MO). SHBG was measured by a chemiluminescent enzyme immunometric assay (DPC; Immulite, Los Angeles, CA), which has an intra-assay CV of <7% and an interassay CV of <8%. Free fatty acids were measured by an enzymatic colorimetric method (WAKO Chemicals, Richmond, VA).
Statistical analysis
Data are presented as means ± SE. Differences in the baseline characteristics of eugonadal and hypogonadal subjects were compared with a two-sample t test for continuous variables; categorical variables were compared using the Fisher’s exact test. A P value <0.05 was considered statistically significant. Pearson’s correlation coefficients were used to assess the relationship between serum concentrations of testosterone and SHBG and measures of adiposity (BMI, WHR, and percent body fat), insulin sensitivity (M), and mitochondrial function (Vo2max, OXPHOS gene expression). Multiple regression analysis was performed to control for potential confounding variables including age, insulin, SHBG, BMI, WHR, and percent body fat. Testosterone levels were analyzed as both a continuous and binary variable (hypogonadal = testosterone <9.7 nmol/l and eugonadal = testosterone ≥9.7 nmol/l). All analyses used study site as a covariate to correct for differences between the Swedish and U.S. cohorts.
RESULTS
The characteristics of the study population are provided in Table 1. Of the subjects, 45% had NGT, 20% had IGT, and 35% had type 2 diabetes. Body composition varied significantly, with a range of BMI from 18.7 to 46.3 kg/m2, WHR from 0.67 to 1.13, and percent body fat from 12.3 to 29.1. Serum testosterone levels covered the spectrum from hypogonadal to eugonadal, ranging from 3 to 31 nmol/l (normal range 9.7-34.6). Testosterone levels were lower in men with IGT and type 2 diabetes than in those with NGT (13.5 ± 6 and 13.1 ± 4 vs. 18 ± 6.5 nmol/l, respectively P < 0.05). The glucose clamp studies revealed a spectrum of insulin sensitivity, with insulin sensitivity values ranging from 1.4 to 13.3 mg · kg−1 · min−1.
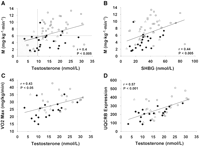
A negative correlation was observed between insulin sensitivity and indexes of obesity, including BMI (r = −0.58, P < 0.0001), WHR (r = −0.6, P < 0.0001), and percent body fat (r = −0.4, P = 0.009). There was an inverse relationship between testosterone and BMI (r = −0.5, P < 0.0001), testosterone and WHR (r = −0.38, P < 0.005), and testosterone and percent body fat (r = −0.5, P < 0.0001). All subjects with hypogonadal testosterone levels had a BMI >25 kg/m2 and a WHR >0.9. An inverse relationship was also observed between SHBG and BMI (r = −0.6, P < 0.001), SHBG and WHR (r = −0.46, P < 0.001), and SHBG and percent body fat (r = −0.4, P = 0.008). A positive correlation was observed between testosterone levels and insulin sensitivity (r = 0.4, P < 0.005; Fig. 1) and between SHBG and insulin sensitivity (r = 0.44, P < 0.005; Fig. 1). In contrast to testosterone, no significant relationship was observed between E2 levels and BMI (r = 0.19), WHR (r = 0.1), or insulin sensitivity (r = 0.14).
As depicted in Fig. 1, the relationship between testosterone levels and insulin sensitivity suggests the presence of two distinct populations. All subjects with a serum testosterone level <9.7 nmol/l had a low insulin sensitivity value. In contrast, men with a low insulin sensitivity value exhibited a full range of serum testosterone levels. Subjects with hypogonadal testosterone levels (n = 10) were more insulin resistant than their eugonadal counterparts (n = 50) (insulin sensitivity = 3.6 ± 0.6 vs. 7.3 ± 3 mg · kg−1 · min−1, respectively; P < 0.0007) (Table 1). Using regression analysis with testosterone as the dependent variable, the association between low testosterone levels and insulin resistance held true after controlling for age, insulin levels, and SHBG (P < 0.05). The relationship between testosterone and insulin sensitivity was no longer significant after adjusting for BMI (P = 0.23), percent body fat (P = 0.17), or WHR (0.38) expressed as continuous variables. However, body composition had less of an impact on this relationship when analyzed using categorical variables, i.e., BMI ≥ or <30 kg/m2 (P = 0.09), body fat ≥ or <25% (P = 0.07), or WHR > or ≤0.9 (P = 0.15). Significant differences were also observed in the metabolic profile of the hypogonadal and eugonadal men (Table 1). Using the definition proposed by the Third National Health and Nutrition Examination Survey (30), 90% of subjects with low testosterone levels met criteria for the metabolic syndrome compared with 29% of those with normal testosterone levels (P < 0.001). Only 1 of 10 subjects with a hypogonadal testosterone level had NGT compared with 26 of 50 eugonadal men.
Expression of OXPHOS-CR genes in skeletal muscle correlated with Vo2max (r = 0.5, P < 0.001) as previously reported (1) and with insulin sensitivity (r = 0.33, P < 0.05). A stronger relationship was observed between the top-ranking OXPHOS-CR gene, UQCRB, and both Vo2max (r = 0.57, P < 0.0001) and insulin sensitivity (r = 0.38, P < 0.05). Testosterone levels correlated positively with both Vo2max (r = 0.43, P < 0.05; Fig. 1) and UQCRB expression (r = 0.57, P < 0.0001; Fig. 1). The relationship between testosterone and expression of the entire OXPHOS-CR gene set was close to statistical significance (r = 0.3, P = 0.08). BMI correlated negatively with Vo2max (r = −0.4, P = 0.009). However, there was no relationship between BMI and expression of either UQCRB (r = −0.18, P = 0.24) or OXPHOS-CR (r = −0.15, P = 0.36). Similarly, a negative correlation was seen between percent body fat and Vo2max (r = −0.49, P = 0.001), and WHR and Vo2max (r = −0.43, P = 0.008). There was a trend for percent body fat to correlate with UQCRB (r = −0.28, P = 0.07), whereas there was no relationship between WHR and UQCRB (r = −0.23, P = 0.16). The relationship between testosterone and Vo2max remained significant when adjusted for BMI (P < 0.05), percent body fat (P < 0.05), and SHBG (P < 0.0001), but not WHR (P = 0.07). Similarly, the relationship between testosterone and UQCRB expression was still significant after adjusting for SHBG levels (P < 0.0001). Finally, no relationship was observed between serum testosterone levels and muscle glycogen content or the percentage of type 1, type 2a, or type 2b muscle fibers.
CONCLUSIONS
These data demonstrate a positive correlation between serum testosterone levels and insulin sensitivity in men across the full spectrum of glucose tolerance. Moreover, men with hypogonadal testosterone levels are twice as insulin resistant as their eugonadal counterparts, and 90% fulfill criteria for the metabolic syndrome. From a clinical perspective, these data highlight the importance of performing a comprehensive metabolic evaluation in men with hypogonadism. The demonstration that testosterone levels correlate not only with insulin sensitivity but also with genetic (OXPHOS gene expression) and functional (Vo2max) markers of mitochondrial function suggests a novel molecular mechanism whereby testosterone might modulate insulin sensitivity in men.
Previous studies on the relationship between androgens and insulin sensitivity in men gave conflicting results depending on whether total or free testosterone levels were used. One explanation for this discrepancy is that SHBG is mediating the link between testosterone and insulin sensitivity. Proponents of the use of free testosterone argue that it is the best index of androgenicity in insulin-resistant men given the low SHBG levels that pertain in this setting (31). An alternative explanation for the discordant results is that the assays used to measure free testosterone have serious methodological limitations, as highlighted in studies validated by mass spectroscopy (32–34). A recent study showed no relationship between free testosterone levels and insulin sensitivity in obese and diabetic men (22). However, the commercial double antibody system used to measure free testosterone in this study correlates poorly with results obtained by equilibrium dialysis (32,35), which is considered the gold standard. In addition, there is controversy as to whether it is only free testosterone that is biologically active, given that testosterone bound to SHBG can bind to cell surface receptors in prostate tissue, leading to activation of adenylyl cyclase and generation of cAMP (36). For these reasons, we chose to use total testosterone levels as the most robust index of androgenicity and to use multiple regression analysis to assess the contribution of SHBG to any association between testosterone levels and insulin sensitivity.
The fact that the correlation between testosterone levels and insulin sensitivity is no longer significant after controlling for BMI could mean that obesity is causing both low testosterone levels and insulin resistance and that there is no direct relationship between testosterone and insulin sensitivity. However, the fact that the impact of BMI on the relationship between testosterone and insulin sensitivity is attenuated significantly when BMI is expressed as a categorical variable suggests that this may not be the case. A second possibility is that the effect of testosterone on insulin sensitivity is mediated through changes in BMI. The relationship between androgens and BMI is likely bi-directional. Morbid obesity has negative effects on the hypothalamic-pituitary-gonadal axis in men (37). In addition, low testosterone levels predispose to central (38) and visceral adiposity (39). In our analysis, all men with hypogonadal testosterone levels have a BMI >25 kg/m2 and a WHR >0.9. Whereas visceral fat was not assessed in the present study, androgens have been shown to have important effects on visceral fat metabolism mediated largely by stimulation of lipolysis. In vitro, testosterone enhances catecholamine-induced lipolysis by increasing the number of β3-adrenergic receptors on rat adipocyte precursor cells (40). Similarly, castration of male rats reduces lipolysis, which is reversed by physiological testosterone replacement (41). In two small studies of men with central obesity, testosterone inhibits lipoprotein lipase activity in abdominal adipose tissue, leading to decreased triglyceride uptake in central fat depots (42,43). Thus, low testosterone levels may predispose to visceral obesity, leading to dysregulation of fatty acid metabolism, which in turn promotes insulin resistance (44).
Although the present study demonstrates a positive correlation between testosterone levels and insulin sensitivity in men, no conclusions can be drawn about causality given the cross-sectional nature of the data. However, in male rats, castration leads to the rapid development of insulin resistance, which is corrected by physiological testosterone replacement (45). In the human, relatively little is known about the impact of androgens on insulin sensitivity. In men with prostate cancer, induction of hypogonadism with a gonadotropin-releasing hormone agonist results in a 60% increase in fasting insulin levels at 3 months (46,47). Data on the impact of androgen supplementation on insulin sensitivity in men are conflicting depending on the population studied. Androgen administration to men with central obesity and testosterone levels in the low normal range increases insulin sensitivity (48–50). However, a recent study of human chorionic gonadotropin (hCG) administration to healthy older men with low normal testosterone levels showed no change in insulin sensitivity (51). Differences in the outcome of this study may reflect the fact that the study population was less obese and that use of human chorionic gonadotropin was associated with very high E2 levels, which may have negated the beneficial effects of androgens. In men with type 2 diabetes, one small nonrandomized study showed no beneficial effect of testosterone replacement on glycemic control (52), whereas a larger non-placebo-controlled study showed a significant reduction in HbA1c (A1C) levels after 3 months of testosterone therapy (53).
Recent studies provide insight into the role of mitochondrial function in the pathogenesis of insulin resistance with the demonstration of decreased expression of peroxisome proliferator-activated receptor-γ coactivator (PGC-1α) and downregulation of OXPHOS genes in skeletal muscle of insulin-resistant subjects (1,2). A model for the pathogenesis of insulin resistance has thus been proposed whereby, in genetically susceptible individuals, environmental risk factors such as physical inactivity contribute to decreased expression of PGC-1α. Reduced PGC-1α expression, in turn, leads to decreased transcription of metabolic and mitochondrial genes and thus decreased oxidative phosphorylation, decreased lipid oxidation, intracellular accumulation of triglycerides in skeletal muscle, and ultimately insulin resistance. In this study, we demonstrate that testosterone levels correlate positively with Vo2max and OXPHOS-CR gene expression. From a mechanistic perspective, little is known about how testosterone might influence insulin action. Induction of hypogonadism with a gonadotropin-releasing hormone (GnRH) agonist in normal men decreases lipid oxidation and resting energy expenditure (54). The insulin resistance resulting from castration in rats is associated with a decrease in glycogen synthase activity (45). Lean offspring of patients with type 2 diabetes (4) have also been shown to have decreased glycogen synthase activity, which has been attributed to intramyocellular triglyceride accumulation. Data from the Otsuka Long Evans Fatty (OLETF) rat, a genetic model of obesity and type 2 diabetes, support a role for androgens in modulating mitochondrial function (55). In this model, expression levels of uncoupling protein 1 (UCP-1) and its upstream regulators, PGC-1α and the β3 adrenergic receptor (β3AR), are reduced, leading to inefficient energy utilization and obesity (55). Administration of dehydroepiandrosterone (DHEA) for 14 days caused a significant increase in UCP-1, β3AR, and PGC-1α expression and reversal of the adverse metabolic phenotype that characterizes the OLETF rat with a reduction in body weight, glucose, insulin, free fatty acids, and leptin levels (55).
Thus, it is plausible that hypogonadism may cause insulin resistance via dysregulation of fatty acid metabolism and that low testosterone levels may represent an additional environmental factor contributing to decreased expression of genes involved in oxidative metabolism. Further studies are required to test this hypothesis by examining gene expression profiles in skeletal muscle before and after androgen manipulations. If testosterone is shown to modulate OXPHOS gene expression, androgen supplementation may represent an important therapeutic modality for preventing or treating the metabolic syndrome and/or type 2 diabetes in men.
Correlation between insulin sensitivity (M) and serum testosterone (T) levels (A) and SHBG levels (B) in 60 men; 27 had NGT ( ) , 12 had IGT (â–µ), and 21 had type 2 diabetes (•). Shaded area represents values for subjects with hypogonadal testosterone levels, i.e., <9.7 nmol/l. In a subset of 42 men, serum testosterone levels were correlated with maximal aerobic capacity (Vo2max) (C) and expression of UQCRB in skeletal muscle (D); 17 men had NGT (), 7 had IGT (â–µ), and 18 had type 2 diabetes (•).
) , 12 had IGT (â–µ), and 21 had type 2 diabetes (•). Shaded area represents values for subjects with hypogonadal testosterone levels, i.e., <9.7 nmol/l. In a subset of 42 men, serum testosterone levels were correlated with maximal aerobic capacity (Vo2max) (C) and expression of UQCRB in skeletal muscle (D); 17 men had NGT (), 7 had IGT (â–µ), and 18 had type 2 diabetes (•).
Clinical and biochemical characteristics of the study population
Acknowledgments
This work was supported by National Institutes of Health Grants K23 DK 02858-04, RO3 DK064276-01, and M01-RR-01066, National Institutes of Health, National Center for Research Resources, General Clinical Research Centers Program. V.K.M. was funded by a physician postdoctoral fellowship from Howard Hughes Medical Institute.
We thank Virginia Hughes for her review of the manuscript and helpful comments.
Footnotes
-
L.G. has served on an advisory panel for Bristol-Myers Squibb.
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
-
- Accepted March 22, 2005.
- Received November 11, 2004.
- DIABETES CARE
References
- ↵
Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, Carlsson E, Ridderstrale M, Laurila E: PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet 34: 267-273, 2003
- ↵
Patti ME, Butte AJ, Crunkhorn S, Cusi K, Berria R, Kashyap S, Miyazaki Y, Kohane I, Costello M, Saccone R: Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proc Natl Acad Sci U S A 100: 8466-8471, 2003
- ↵
Petersen KF, Befroy D, Dufour S, Dziura J, Ariyan C, Rothman DL, DiPietro L, Cline GW, Shulman GI: Mitochondrial dysfunction in the elderly: possible role in insulin resistance Science 300: 1140-1142, 2003
- ↵
Petersen KF, Dufour S, Befroy D, Garcia R, Shulman GI: Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Engl J Med 350: 664-671, 2004
- ↵
Wisloff U, Najjar SM, Ellingsen O, Haram PM, Swoap S, Al-Share Q, Fernstrom M, Rezaei K, Lee SJ, Koch LG, Britton SL: Cardiovascular risk factors emerge after artificial selection for low aerobic capacity. Science 307: 418-420, 2005
- ↵
Dunaif A: Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr Rev 18: 774-800, 1997
- ↵
Phillips GB: Relationship between serum sex hormones and glucose, insulin and lipid abnormalities in men with myocardial infarction. Proc Natl Acad Sci U S A 74: 1729-1733, 1977
-
Lichtenstein MJ, Yarnell JW, Elwood PC, Beswick AD, Sweetnam PM, Marks V, Teale D, Riad-Fahmy D: Sex hormones, insulin, lipids, and prevalent ischemic heart disease. Am J Epidemiol 126: 647-657, 1987
-
Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R: Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism 39: 897-901, 1990
-
Pasquali R, Casimirri F, Cantobelli S, Melchionda N, Morselli Labate AM, Fabbri R, Capelli M, Bortoluzzi L: Effect of obesity and body fat distribution on sex hormones and insulin in men. Metabolism 40: 101-104, 1991
- ↵
Simon D, Preziosi P, Barrett-Connor E, Roger M, Saint-Paul M, Nahoul K, Papoz L: Interrelation between plasma testosterone and plasma insulin in healthy adult men: the Telecom Study. Diabetologia 35: 173-177, 1992
- ↵
Barrett-Connor E: Lower endogenous androgen levels and dyslipidemia in men with non-insulin-dependent diabetes mellitus. Ann Intern Med 117: 807-811, 1992
- ↵
Andersson B, Marin P, Lissner L, Vermeulen A, Bjorntorp P: Testosterone concentrations in women and men with NIDDM. Diabetes Care 17: 405-411, 1994
- ↵
Tibblin G, Adlerberth A, Lindstedt G, Bjorntorp P: The pituitary-gonadal axis and health in elderly men: a study of men born in 1913. Diabetes 45: 1605-1609, 1996
-
Haffner SM, Shaten J, Stern MP, Smith GD, Kuller L: Low levels of sex hormone-binding globulin and testosterone predict the development of non-insulin-dependent diabetes mellitus in men. Am J Epidemiol 143: 889-897, 1996
-
Stellato RK, Feldman HA, Hamdy O, Horton ES, McKinlay JB: Testosterone, sex hormone-binding globulin, and the development of type 2 diabetes in middle-aged men. Diabetes Care 23: 490-494, 2000
-
Oh J-Y, Barrett-Connor E, Wedick NM, Wingard DL: Endogenous sex hormones and the development of type 2 diabetes in older men and women: the Rancho Bernardo Study. Diabetes Care 25: 55-60, 2002
-
Svartberg J, Jenssen T, Sundsfjord J, Jorde R: The associations of endogenous testosterone and sex hormone-binding globulin with glycosylated hemoglobin levels, in community dwelling men: the Tromso Study. Diabetes Metab 30: 29-34, 2004
- ↵
Laaksonen DE, Niskanen L, Punnonen K, Nyysonen K, Tuomainen T-P, Valkonen V-P, Salonen R, Salonen JT: Testosterone and sex hormone-binding globulin predict the metabolic syndrome and diabetes in middle-aged men. Diabet es Care 27: 1036-1041, 2004
- ↵
Haffner SM, Karhapaa P, Mykkanen L, Laakso M: Insulin resistance, body fat distribution, and sex hormones in men. Diabetes 43: 212-219, 1994
- ↵
Birkeland KI, Hanssen KF, Torjesen PA, Vaaler S: Level of sex hormone-binding globulin is positively correlated with insulin sensitivity in men with type 2 diabetes. J Clin Endocrinol Metab 76: 275-278, 1993
- ↵
Abate N, Haffner SM, Garg A, Peshock RM, Grundy SM: Sex steroid hormones, upper body obesity, and insulin resistance. J Clin Endocrinol Metab 87: 4522-4527, 2002
- ↵
Morley JE, Kaiser FE, Perry HM 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ: Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 46: 410-413, 1997
-
Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR: Longitudinal effects of aging on serum total and free testosterone levels in healthy men: Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab 86: 724-731, 2001
- ↵
Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, Bremner WJ, McKinlay JB: Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab 87: 589-598, 2002
- ↵
Eriksson KF, Lindgarde F: Impaired glucose tolerance in a middle-aged male urban population: a new approach for identifying high-risk cases. Diabetologia 33: 526-531, 1990
- ↵
World Health Organization: Diabetes Mellitus: Report of a WHO Study Group. Geneva, World Health Org., 1985 (Tech. Rep. Ser., no. 727)
- ↵
DeFronzo RA, Tobin JD, Andres R: Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol 237: E214-E223, 1979
- ↵
Schalin-Jantti C, Laurila E, Lofman M, Groop LC: Determinants of insulin-stimulated skeletal muscle glycogen metabolism in man. Eur J Clin Invest 25: 693-698, 1995
- ↵
Ford ES, Giles WH, Dietz WH: Prevalence of the metabolic syndrome among US adults: findings from the Third National Health and Nutrition Examination Survey. JAMA 287: 356-359, 2002
- ↵
Plymate SR, Matej LA, Jones RE, Friedl KE: Inhibition of sex hormone-binding globulin production in the human hepatoma (Hep G2) cell line by insulin and prolactin. J Clin Endocrinol Metab 67: 460-464, 1988
- ↵
Miller KK, Rosner W, Lee H, Hier J, Sesmilo G, Schoenfeld D, Neubauer G, Klibanski A: Measurement of free testosterone in normal women and women with androgen deficiency: comparison of methods. J Clin Endocrinol Metab 89: 525-533, 2004
-
Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS: Measurement of total serum testosterone in adult men: comparison of current laboratory methods versus liquid chromatography-tandem mass spectrometry. J Clin Endocrinol Metab 89: 534-543, 2004
- ↵
Matsumoto AM, Bremner WJ: Serum testosterone assays: accuracy matters (Editorial). J Clin Endocrinol Metab 89: 520-523, 2004
- ↵
Vermeulen A, Verdonck L, Kaufman JM: A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666-3672, 1993
- ↵
Rosner W, Hyrb DJ, Khan MS, Nakhla AM, Romas, NA: Androgen and estrogen signaling at the cell membrane via G-proteins and cyclic adenosine monophosphate. Steroids 64: 100-106, 1999
- ↵
Giagulli VA, Kaufman JM, Vermeulen A: Pathogenesis of the decreased androgen levels in obese men. J Clin Endocrinol Metab 79: 997-1000, 1994
- ↵
Khaw KT, Barrett-Connor E: Lower endogenous androgens predict central adiposity in men. Ann Epidemiol 2: 675-682, 1992
- ↵
Tsai EC, Boyko EJ, Leonetti DL, Fujimoto WY: Low serum testosterone level as a predictor of increased visceral fat in Japanese-American men. Int J Obes Relat Metab Disord 4: 485-491, 2000
- ↵
Xu XF, De Pergola G, Bjorntorp P: The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinolog y 126: 1229-1234, 1990
- ↵
Xu XF, De Pergola G, Bjorntorp P: Testosterone increases lipolysis and the number of beta-adrenoreceptors in male rat adipocytes. Endocrinology 128: 379-382, 1991
- ↵
Rebuffe-Scrive M, Marin P, Bjorntorp P: Effect of testosterone on abdominal adipose tissue in men. Int J Obes 15: 791-795, 1991
- ↵
Marin P, Oden B, Bjorntorp P: Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J Clin Endocrinol Metab 80: 239-243, 1995
- ↵
Boden G, Jadali F, White J, Liang Y, Mozzoli M, Chen X, Coleman E, Smith C: Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest 88: 960-966, 1991
- ↵
Holmang A, Bjorntorp P: The effects of testosterone on insulin sensitivity in male rats. Acta Physiol Scand 146: 505-510, 1992
- ↵
Smith JC, Bennett S, Evans LM, Kynaston HG, Parmar M, Mason MD, Cockcroft JR, Scanlon MF, Davies JS: The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab 86: 4261-4267, 2001
- ↵
Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C: Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinemia. Clin Sci 104: 195-201, 2003
- ↵
Marin P, Holmang S, Jonsson L, Sjostrom L, Kvist H, Holm G, Lindstedt G, Bjorntorp P: The effects of testosterone treatment on body composition and metabolism in middle-aged obese men. Int J Obes 16: 991-997, 1992
-
Marin P, Holmang S, Gustafsson C, Jonsson L, Kvist H, Elander A, Eldh J, Sjostrom L, Bjorntorp P: Androgen treatment of abdominally obese men. Obes Res 1: 245-251, 1993
- ↵
Simon D, Charles MA, Lahlou N, Nahoul K, Oppert JM, Gouault-Heilmann M, Lemort N, Thibult N, Joubert E, Balkau B, Eschwege E: Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone: a 3-month randomized placebo-controlled trial. Diabetes Care 24: 2149-2151, 2001
- ↵
Liu PY, Wishart SM, Celermajer DS, Jimenez M, Pierro ID, Conway AJ, Handelsman DJ: Do reproductive hormones modify insulin sensitivity and metabolism in older men? A randomized, placebo-controlled clinical trial of recombinant human chorionic gonadotropin. Eur J Endocrinol 148: 55-66, 2003
- ↵
Corrales JJ, Burgo RM, Garca-Berrocal B, Almeida M, Alberca I, Gonzalez-Buitrago JM, Orfao A, Miralles JM: Partial androgen deficiency in aging type 2 diabetic men and its relationship to glycemic control. Metabolism 53: 666-672, 2004
- ↵
Boyanov MA, Boneva Z, Christov VG: Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6: 1-7, 2003
- ↵
Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ: Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab 83: 1886-1892, 1998
- ↵
Ryu JW, Kim MS, Kim CH, Song KH, Park JY, Lee JD, Kim JB, Lee KU: DHEA administration increases brown fat uncoupling protein 1 levels in obese OLETF rats. Biochem Biophys Res Commun 303: 726-731, 2003
Articles citing this article
-
Type 2 Diabetes in the Pediatric Population: Are We Meeting ADA Clinical Guidelines in Ohio? Clinical Pediatrics April 1, 2010 49:316-322
-
Low testosterone and sex hormone-binding globulin levels and high estradiol levels are independent predictors of type 2 diabetes in men European Journal of Endocrinology April 1, 2010 162:747-754
-
Fifty-two-Week Treatment With Diet and Exercise Plus Transdermal Testosterone Reverses the Metabolic Syndrome and Improves Glycemic Control in Men With Newly Diagnosed Type 2 Diabetes and Subnormal Plasma Testosterone Journal of Andrology November 1, 2009 30:726-733
-
Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: the Health In Men Study European Journal of Endocrinology October 1, 2009 161:591-598
-
Mitochondrial Complex I Impairment in Leukocytes from Polycystic Ovary Syndrome Patients with Insulin Resistance Journal of Clinical Endocrinology & Metabolism September 1, 2009 94:3505-3512
-
The Emerging Link Between Hypogonadism and Metabolic Syndrome Journal of Andrology July 1, 2009 30:370-376
-
Potential Side Effects of Androgen Deprivation Treatment in Sex Offenders Journal of the American Academy of Psychiatry and the Law Online March 1, 2009 37:53-58
-
The Dark Side of Testosterone Deficiency: II. Type 2 Diabetes and Insulin Resistance Journal of Andrology January 1, 2009 30:23-32
-
Testosterone Concentration in Young Patients With Diabetes Diabetes Care October 1, 2008 31:2013-2017
-
Relationship Between Serum Testosterone and Cardiovascular Risk Profile in the General Population Archives of Internal Medicine June 23, 2008 168:1350
-
Lower sex hormone-binding globulin is more strongly associated with metabolic syndrome than lower total testosterone in older men: the Health in Men Study European Journal of Endocrinology June 1, 2008 158:785-792
-
Acute Sex Steroid Withdrawal Reduces Insulin Sensitivity in Healthy Men with Idiopathic Hypogonadotropic Hypogonadism Journal of Clinical Endocrinology & Metabolism November 1, 2007 92:4254-4259
-
Alcohol Consumption and Type 2 Diabetes: Influence of Genetic Variation in Alcohol Dehydrogenase Diabetes September 1, 2007 56:2388-2394
-
Reversal of Idiopathic Hypogonadotropic Hypogonadism New England Journal of Medicine August 30, 2007 357:863-873
-
The Effects of Hypogonadism on Body Composition and Bone Mineral Density in Type 2 Diabetic Patients Diabetes Care July 1, 2007 30:1860-1861
-
Associations of Total Testosterone and Sex Hormone-Binding Globulin Levels With Insulin Sensitivity in Middle-Aged Finnish Men Diabetes Care April 1, 2007 30:e13
-
Effects of Testosterone Supplementation on Whole Body and Regional Fat Mass and Distribution in Human Immunodeficiency Virus-Infected Men with Abdominal Obesity Journal of Clinical Endocrinology & Metabolism March 1, 2007 92:1049-1057
-
Cardiopulmonary Impairment in Young Women with Polycystic Ovary Syndrome Journal of Clinical Endocrinology & Metabolism August 1, 2006 91:2967-2971
-
Serum adiponectin and leptin levels in relation to the metabolic syndrome, androgenic profile and somatotropic axis in healthy non-diabetic elderly men. European Journal of Endocrinology July 1, 2006 155:167-176
-
Sex Differences of Endogenous Sex Hormones and Risk of Type 2 Diabetes: A Systematic Review and Meta-analysis JAMA March 15, 2006 295:1288-1299
-
Relationship between testosterone levels, insulin sensitivity, and mitochondrial function in men. Diabetes Care March 1, 2006 29:749
-
Relationship Between Testosterone Levels, Insulin Sensitivity, and Mitochondrial Function in Men: Response to Kaplan and Crawford Diabetes Care March 1, 2006 29:749-750


 CiteULike
CiteULike Del.icio.us
Del.icio.us Digg
Digg Reddit
Reddit Technorati
Technorati Facebook
Facebook Twitter
Twitter