Dr. Weeks’ Comment: For the past 7 years, I have been lecturing to doctors and urging oncologists to focus on addressing the cancer STEM cells – those cancer cells which are actually dangerous (they are the ones which metastasize and which are resistant to chemotherapy and radiation). Now the news is getting out. Ask your oncologist what he or she is planning to do about your cancer STEM cells…
Cancer Stem Cells in the Crosshairs
Investors are showing mounting confidence in startup biotechnology firms preparing therapies directed against cancer stem cells.
Startup firms are quietly launching clinical trials focused against cells that may drive tumor formation and drug resistance
It’s shaping up as a good year for biotechnology firms that focus on cancer stem cells. In January, Verastem, a stem cell startup in Cambridge, MA, raised $57 million in its initial public offering of stock, despite being a year away from clinical trials. In February, the Japanese pharmaceutical giant Dainippon Sumitomo bought Boston Biomedical Inc.””a Norwich, MA-based biotech with 2 anticancer stem cell agents in clinical trials””for $200 million down and nearly $2.4 billion in milestone payments.
Both events signal mounting investor confidence in therapies directed against cancer stem cells””self-renewing cells that are implicated in metastasis and drug resistance and can be detected in human tumors, including those of the blood, breast, brain, head and neck, prostate, colon, and other tissues.
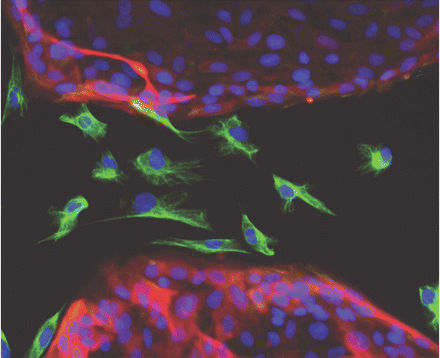
Passage through an epithelial-to-mesenchymal transition (EMT) is a major step in the formation of cancer stem cells, according to research by Robert Weinberg, PhD, of Whitehead Institute for Biomedical Research, and many other scientists. Weinberg is among the founders of Verastem, a company formed to develop inhibitors of cancer stem cells. During EMT, static, tightly packed epithelial cells (highlighted in red with blue nuclei) acquire the traits of freely moving, loose mesenchymal cells (highlighted in green with blue nuclei). [Image courtesy Christina Scheel/Whitehead Institute for Biomedical Research]
Much about these cells has been controversial, and much remains unknown. “We’re learning that they have a degree of plasticity,” says Jenny Chang, MD, director of the Methodist Cancer Center at Methodist Hospital in Houston, TX, whose research includes studies on breast cancer stem cells. “They can go from being tumor-initiating cells to more differentiated cancer cells and back again; it’s not just one fixed population that never changes.”
As many labs grapple for a better understanding of cancer stem cells, a growing number of cancer stem cell therapies are in clinical development.
On Pathways to “Stemness”
Boston Biomedical’s lead compound, BBI608, a small-molecule agent, is now being readied for phase III clinical trials in colorectal cancer this year in North America, in addition to phase Ib and II trials in ovarian, breast, non-small cell lung, melanoma, head and neck, and other solid tumors.
The drug’s specific molecular target hasn’t been publicly disclosed. The company’s founder and chief executive officer, Chiang J. Li, MD, would say only that binding the target inhibits “cancer cell “stemness” pathways that include those induced by c-myc, STAT3, and β-catenin. Results from earlier trials have not been published.
Boston Biomedical’s second lead candidate, another small molecule, is BBI503, a cancer stemness kinase inhibitor that homes in on yet another undisclosed target. This agent is headed for phase I trials in solid tumors in the United States later this year.
While Boston Biomedical tailors its drugs to their intended targets, Verastem relies on high-throughput screening using cancer cells induced to express stem-like properties. Developed in 2009 by researchers at the Whitehead and Broad Institutes in Cambridge, MA, this assay has since been used to evaluate 320,000 compounds for cancer stem-cell killing effects, says Verastem’s head of research, Jonathan Pachter, PhD.
Three small molecules have emerged as Verastem’s top contenders. They include VS-507, which targets the Wnt/β-catenin signaling pathway and is expected to go into clinical trials late this year, and 2 other compounds””VS-4718 and VS-5095””that target focal adhesion kinase, or FAK. “Our main focus right now is to develop these agents against triple-negative breast cancer,” Pachter says.
OncoMed Pharmaceuticals, a biotech in Redwood City, CA, has placed its bets on Wnt and Notch signaling, which are both linked to cancer stem cell proliferation. The company’s lead product””OMP-21M18, now in phase Ib/II clinical trials for various solid tumors””targets Notch specifically and pushes cancer stem cells toward a more differentiated state, according to OncoMed’s chief science officer, John Lewicki, PhD. This heightens their vulnerability to chemotherapy, Lewicki says, suggesting that OMP-21M18 might be most effective in combination treatment with other drugs.
Some big pharmaceutical firms also are focusing in this area. Pfizer, for instance, is exploring ways to target 5T4, a cell-surface protein that appears to be upregulated in cancer stem cells. But because small biotech companies can develop the needed technologies faster, they dominate cancer stem cell research, comments Hans-Peter Gerber, PhD, executive director of oncology research at Pfizer.
Hitting Targets, Avoiding Toxicity
It’s not clear what targets will render cancer stem cells most vulnerable to attack, according to Gerber. “This is still an open question,” he says. “They could be on or within the cells or they could be in the cell’s microenvironment.”
A major challenge, Gerber adds, is to ensure that the drugs don’t also target normal stem cells that replenish damaged tissues. “We’re finding that the signaling pathways that cancer stem cells rely on can be similar to the pathways that control embryonic and adult tissue development,” he says. Still, Gerber emphasizes that, so far, cancer stem cells seem to have more in common with tumor cells than they do with stem cells in healthy tissues.
Chang notes that this supports the notion that drugs can be developed to selectively attack stem cells in tumors. “But given their plasticity, some more differentiated cells might go back to being stem-like after treatment,” she adds. “So it’s likely that we’ll need drug combinations and repeat treatments to kill cancer stem cells off entirely.”