Dr. Weeks’ Comment: My friend and colleague Uwe pointed out 8 years ago that the MAJORITY of animal research in cancer DOES NOT APPLY to humans because the estrogen metabolism of mice is radially different from that of humans. This was UNWELCOME news, to say the least revealing as it did, years of erroneous research and the consequential untold suffering by cancer patients. Oncologists should now understand that the mouse studies are not an ideal model for extrapolation to humans.
`
Deceptive model: Stem cells of humans and mice differ more strongly than suspected
March 5, 2010 Enlarge
Enlarge
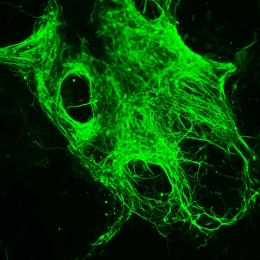
Neural differentiation of mouse epiblast stem cells by inactivating the FGF signalling pathway. Molecular mechanisms to differentiate human and mouse stem cells may be similar but may also vary substantially on occasion. Image: Boris Greber
(PhysOrg.com) — They are considered to be the most important model organism for research into human biology: mice may look totally different, but they are in many ways similar to Homo sapiens on a fundamental level.
For instance, an impressive 99 per cent of the mouse genes are matched by a corresponding sequence in the human genome. That is also why the law in this part of the world only permits scientists to conduct research on human embryo stem cells when they have “clarified in advance” their specific questions by using animal cells as far as possible. However, such tests are often pointless – and sometimes even misleading, as a recent study by scientists working with Hans Schöler at the Max Planck Institute for Molecular Biomedicine in Münster demonstrates. (Cell Stem Cell, March 5th, 2010)
For years scientists have puzzled over to what extent the findings of studies on the embryonic stem cells (ES cells) of mice are transferable to humans. It is certainly true that human and mouse ES cells are both pluripotent. That means they are capable of forming any of the body’s cell types, numbering more than 200 in all. And both types of cells have an active Oct4 transcription factor, for example. This is the gene that is essential for maintaining pluripotency, and is what makes egg cells, as well as embryonic stem cells and early embryos, potentially immortal.
In other aspects, though, as scientists have known for some time now, human and mouse ES cells differ enormously. Certain signalling substances that can be used to turn mouse cells into liver, nerve or muscle cells, for instance, produce either no effect or totally different effects in human ES cells.
The reasons for this are still uncertain. However, in 2007 two research teams succeeded in isolating a promising new type of pluripotent cells from mice embryos (see Brons et al., Nature 448, 2007). Known as epiblast stem cells (EpiSC), these cells are also pluripotent. However, they stem from a later stage of embryonic development: unlike ”˜traditional’ ES cells, which are harvested from a few-days-old embryo in the blastocyst stage, these are harvested from an embryo that has just lodged itself in the uterus and which is referred to as an epiblast.
The astonishing thing about it is that although epiblast stem cells are actually a step ahead in their development, they appear to be more similar to human ES cells than ”˜classic’ mouse ES cells are. For example, both epiblast stem cells and human ES cells can, with the addition of a certain hormone, the FGF2 growth factor, be grown and held in a state in which they can turn into any tissue at all. “Epiblast stem cells from mice are therefore more-or-less equated with human ES cells in the general scientific discussion,” says Boris Greber, the lead author of the study.
Differing effects of signal molecules
But Greber, a biochemist, wanted to know more. In their latest study, he and his fellow scientists therefore looked at how mouse epiblast and humanembryonic stem cells react to different growth factors and inhibitors – and they found that the two types of cells do, in fact, differ on a crucial point. Whereas the FGF growth factor actively supports the self-renewal of human ES cells, this is not the case with mouse epiblast cells.
“Ultimately, what this means is that many preliminary tests on animal cells – particularly in medically relevant projects – may not only be useless, but the findings from this kind of early testing may even be misleading,” explains Hans Schöler, who goes on to say that human ES cells will therefore continue to be absolutely essential for stem cell research in the future. “The recent successes in reprogramming mature human somatic cells sometimes make it look as though tests using human ES cells are nowadays redundant. But appearances are deceptive.” Neither the technologies for reprogramming nor those for purposefully differentiating cells are as yet fully-developed.
Human stem cells remain indispensable
Only a fraction of the cells that the scientists treat with their formulas go on to display the right attributes. And only through elaborate, time-consuming tests can the successfully transformed cells be picked out from among the large numbers of cells that failed to be completely reprogrammed. “Our latest study demonstrates that animal model systems are inadequate for a great many tests of this kind,” says Schöler. “Particularly when we’re talking about developing safe and effective stem cell therapies, we will still need human ES cells as the gold standard against which to compare everything else. In such cases, lengthy preliminary testing on animal cells risks wasting valuable time and resources.”
More information: Conserved and divergent roles of FGF signaling in mouse epiblast stem cells and human embryonic stem cells, Cell Stem Cell, 5 March 2010, doi:10.1016/j.stem.2010.01.003
Provided by Max-Planck-Gesellschaft (news : web)
`
`