Dr. Weeks’ Comment: While I lecture about the importance to doctoring cancer STEM cells around the country, my neighbors 30 minutes to the south at the University of Washington are using the the astonishing embryonic stem cells to push forward the frontiers of medicine: here we learn about the injection of stem cells to repair the heart.
Stem cell therapy regenerates heart muscle damaged from heart attacks in primates
Heart cells created from human embryonic stem cells successfully restored damaged heart muscles in monkeys.
The results of the experiment appear in the April 30 advanced online edition of the journal Nature in a paper titled, “Human embryonic-stem cell derived cardiomyocytes regenerate non-human primate hearts.”
The findings suggest that the approach should be feasible in humans, the researchers said.
“Before this study, it was not known if it is possible to produce sufficient numbers of these cells and successfully use them to remuscularize damaged hearts in a large animal whose heart size and physiology is similar to that of the human heart,” said Dr. Charles Murry, UW professor of pathology and bioengineering, who led the research team that conducted the experiment.
A physician/scientist, Murry directs the UW Center for Cardiovascular Biology and is a UW Medicine pathologist.
Murry said he expected the approach could be ready for clinical trials in humans within four years.
In the study, Murry, along with Dr. Michael Laflamme and other colleagues at the UW Institute for Stem Cell & Regenerative Medicine, experimentally induced controlled myocardial infarctions, a form of heart attack, in anesthetized pigtail macaques.
The infarcts were created by blocking the coronary artery of macaque for 90 minutes, an established model for the study of myocardial infarction in primates.
In humans, myocardial infarctions are typically caused by coronary artery disease. The resulting lack of adequate blood flow can damage heart muscle and other tissues by depriving them of oxygen. Because the infarcted heart muscle does not grow back, myocardial infarction leaves the heart less able to pump blood and often leads to heart failure, a leading cause of cardiovascular death.
The goal of stem cell therapy is to replace the damaged tissue with new heart cells and restore the failing heart to normal function.
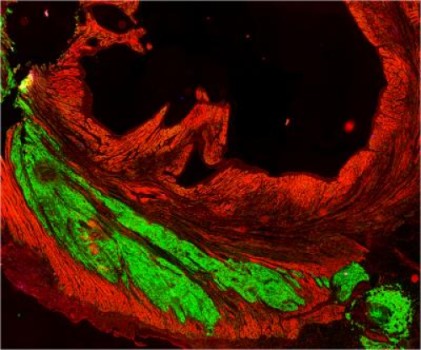
Two weeks after the experimental myocardial infarctions, the Seattle researchers injected 1 billion heart muscle cells derived from human embryonic stem cells, called human embryonic stem cell-derived cardiomyocytes, into the infarcted muscle. This was ten times more of these types of cells than researchers have ever been able to generate before.
All the monkeys had been put on immunosuppressive therapy to prevent rejection of the transplanted human cells.
The researchers found that over subsequent weeks, the stem-cell derived heart muscle cells infiltrated into the damaged heart tissue, then matured, assembled into muscle fibers and began to beat in synchrony with the macaque heart cells. After three months, the cells appear to have fully integrated into the macaque heart muscle.
On average the transplanted stem cells regenerated 40 percent of the damaged heart tissue, said Dr. Michael Laflamme, UW assistant professor of pathology, whose team was principally responsible for generating the replacement heart muscle cells.
“The results show we can now produce the number of cells needed for human therapy and get formation of new heart muscle on a scale that is relevant to improving the function of the human heart,” Laflamme said.
Ultrasound studies of the macaques’ hearts showed that the ejection fraction, an indication of the hearts ability to pump blood, improved in some of the treated animals but not all. The researchers also found that arteries and veins from the macaques’ hearts grew into the new heart tissue, the first time it has been shown that blood vessels from a host animal will grow into and nurture a large stem-cell derived graft of this type.
The most concerning complications were episodes of irregular heartbeats, or arrhythmias, that occurred in the weeks after the macaques received the stem cell injections, Murry said. None of the macaques, however, appeared to have symptoms during these episodes, which disappeared after two to three weeks as the stem cells matured and became more electrically stable.
Going forward the UW researchers will work to reduce the risk of arrhythmias, perhaps by using more electrically mature stem cells. They also will try to demonstrate definitively that the stem cells are actually strengthening the heart’s pumping power.
These cells have improved the mechanical function in every other species in which they have been tested, so we are optimistic they will do so in this model as well,” Murry said.
Journal Reference:
- James J. H. Chong, Xiulan Yang, Creighton W. Don, Elina Minami, Yen-Wen Liu, Jill J. Weyers, William M. Mahoney, Benjamin Van Biber, Nathan J. Palpant, Jay A. Gantz, James A. Fugate, Veronica Muskheli, G. Michael Gough, Keith W. Vogel, Cliff A. Astley, Charlotte E. Hotchkiss, Audrey Baldessari, Lil Pabon, Hans Reinecke, Edward A. Gill, Veronica Nelson, Hans-Peter Kiem, Michael A. Laflamme, Charles E. Murry. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature, 2014; DOI:10.1038/nature13233