Dr. Weeks’ Comment: OK, you have breast cancer. But has your doctor told you about cancer STEM cells and how “many cancers, including breast cancer, are driven by a cellular subpopulation that displays stem cell properties. These cells may mediate tumor metastasis and by virtue of their relative resistance to conventional therapies contribute to relapse.” And has your doctor measured your IL-8 levels to determine your risk of recurrence? Did he or she describe to you the “correlation between levels of IL-8 in metastatic fluids and BCSCs frequently support previous studies showing a correlation between serum levels of IL-8 and poor patient outcome in patients with metastatic breast cancer.” Has your oncologist measured your ALDH1 which has been shown to be an independent predictor of poor outcome in breast cancer?
Sadly, none of these have been tested because (work with me here…) the treatment is an inexpensive anti-inflammatory agent. (That is bad for the business of Big Pharma) Also, sorry to say, your oncologist can’t offer you the best treatment, only the standard of care is offered and, despite research clearly warning that “chemotherapy and radiation make your cancer worse” that is all they are allowed to offer you – unless they want to risk their medical license in order to save your life with tomorrow’s medicine today. Apoptosis is appropriate cell death. That is the goal of the standard of care for cancer. Apoptosis is the goal of chemotherapy and radiation but will it help? Sadly NO. According to Dr. Efimova et al “Apoptosis induced by chemotherapeutic agents may also increase intratumoral levels of IL-8 stimulating the cancer stem cell population” .
Breast Cancer Stem Cells: We’ve Got Them Surrounded
- Hasan Korkaya and Max S. Wicha Clin Cancer Res; 19(3); 511-3. ©2012 AACR.
Abstract
Breast cancer stem cells are regulated by cell intrinsic pathways as well as by elements in the tumor microenvironment. New evidence suggests that an important interaction between the interleukin (IL)-8 receptor CXCR1/2 and HER2 is involved in this regulation. Simultaneous targeting of these pathways provides a novel therapeutic approach.
In this issue of Clinical Cancer Research, Singh and colleagues provide new insights into the mechanisms that regulate breast cancer stem cells (BCSC; ref. 1). There is now substantial evidence that many cancers, including breast cancer, are driven by a cellular subpopulation that displays stem cell properties. These cells may mediate tumor metastasis and by virtue of their relative resistance to conventional therapies contribute to relapse. An understanding of the pathways that regulate BCSCs is, thus, of great importance and may facilitate the development of agents capable of targeting these cell populations.
Previous studies have suggested that BCSCs are regulated by intrinsic cellular pathways as well as extrinsic signals generated by the tumor microenvironment (2). Studies by Singh and colleagues in this issue further our understanding of these pathways by showing interactions between the IL-8/CXCR1/2 axis and HER2 signaling in the regulation of BCSCs. These studies build upon prior work showing independent roles for these pathways in regulating the self-renewal of BCSCs. Using gene expression profiling, our group previously identified CXCR1 as being overexpressed in cancer cells expressing the stem cell marker aldehyde dehydrogenase (ALDH) in a series of breast cancer cell lines (3). CXCR1 is a receptor for the cytokine interleukin-8 (IL-8), and we showed that recombinant IL-8 increased BCSC self-renewal as determined by the ability of these cells to form tumor spheres as well as by increased ALDH expression.
Singh and colleagues show the clinical importance of IL-8 by directly measuring IL-8 levels in plural effusions and ascites from 10 patients with metastatic breast cancer. Of interest, they show a clear association between metastatic fluid IL-8 levels and ability of cells isolated from these effusions to generate primary and secondary tumor spheres. This provides important clinical validation for the importance of IL-8 in regulating BCSCs. IL-8 is produced by a variety of cells within the tumor microenvironment including cancer-associated fibroblasts and cytokine-mediated immune cells. Paracrine interactions between these cell populations may thus regulate tumor growth at metastatic sites.
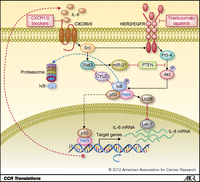
Approximately 20% of breast cancers display amplification of the HER2 gene, a genotype associated with aggressive course and poor clinical outcome (4). The development of HER2-targeting agents such as trastuzumab and lapatinib represents one of the greatest achievements in clinical oncology with significant benefits when these agents are administered in both adjuvant and advanced disease settings. Our group and others have shown that HER2 is an important intrinsic regulator of BCSCs (5, 6). This regulation occurs through activation of the Wnt/b-catenin pathway via Akt-mediated phosphorylation of GSK3B and nuclear translocation of β-catenin (7). Furthermore, resistance to HER2-targeting agents such as trastuzumab may be driven by PTEN deletion through activation of an inflammatory loop involving NF-κB, which in turn can regulate the production of cytokines including IL-6 and IL-8 (8). Interestingly, Singh and colleagues now show an interaction between CXCR1/2 signaling and activation of the HER2 pathway. This seems to occur through CXCR1/2 activation of Src, which in turn phosphorylates HER2-activating downstream signaling cascades as illustrated in Fig. 1.
The EGFR/HER2 inhibitor lapatinib inhibits the tumorsphere promoting effects of IL-8 in both HER2-positive and -negative patient-derived cancers. This suggests that CXCR1/2 signals through both HER2-dependent and -independent pathways. In addition, it shows an important role for EGFR/HER2 signaling in a wider group of breast cancers than those that display HER2 amplification. IL-8 stimulation of cells led to rapid phosphorylation of Akt and extracellular signal-regulated kinase (ERK). Although lapatinib pretreatment completely blocked the IL-8-mediated transactivation of phospho-HER2 and phospho-ERK1/2, it only partially inhibited the effect of IL-8 on Akt phosphorylation. Consistent with our previous findings, IL-8 induces the self-renewal by activating Akt signaling (3). The importance of both Akt and ERK1/2 signaling cascades in regulating BCSC self-renewal was confirmed by showing that inhibitors of these pathways blocked sphere formation.
Furthermore, the Src inhibitor PP2 inhibited IL-8-induced HER2, Akt, and ERK1/2 phosphorylation and reduced tumor sphere formation. These findings are consistent with previous studies showing that Src stimulates transcriptional activation of NF-κB via Stat3/CYLD signaling cascade leading to the generation of an inflammatory feedback loop (9) as shown in Fig. 1. The studies of Singh and colleagues suggest that in addition to the previously described inflammatory feedback loop involving NF-κB and IL-6 there exists an additional positive feedback loop involving IL-8 and HER2. A recent report showing that HER2/HER3 activity leads to overexpression of IL-8 (10), combined with the reports of Singh and colleagues, supports the existence of such a positive feedback loop.
Targeting HER2-positive BCSCs by dual blockade of HER2 and CXCR1/2 signaling pathways. Elevated levels of IL-8 drives EGFR/HER2 transactivation as well as activation of the Src/Stat3/NF-κB pathway. Combined inhibition of CXCR1/2 and EGFR/HER2 inhibits CSC self-renewal in HER2-positive breast cancer.
The studies of Singh and colleagues also have important clinical implications. Their data showing a correlation between levels of IL-8 in metastatic fluids and BCSCs frequently support previous studies showing a correlation between serum levels of IL-8 and poor patient outcome in patients with metastatic breast cancer (11). Apoptosis induced by chemotherapeutic agents may also increase intratumoral levels of IL-8 stimulating the cancer stem cell population (12). We previously have shown that reparaxin, a small-molecule inhibitor of CXCR1/2, inhibits BCSC in mouse xenografts (3). On the basis of this, we have initiated a phase I clinical trial combining reparaxin with chemotherapy in women with advanced breast cancers. The studies of Singh and colleagues suggest that combination of HER2-blocking agents may synergize with CXCR1/2 inhibitors in targeting the BCSC population. The recent developments of HER2-blocking agents with increased clinical efficacy such as T-DM1 may provide highly potent agents to test such approaches. The simultaneous targeting of interacting extrinsic and intrinsic CSC regulatory pathways may result in more efficient targeting of BCSC populations improving patient outcome.