|
The Management of Estrogens, Estrogen Receptors, Estrogen Metabolism, and Cellular Immunity in the Treatment of Cancers |
||||||||||||||
|
|
||||||||||||||
|
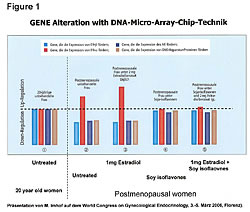
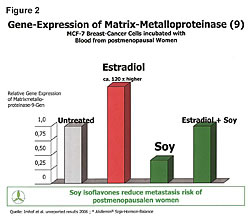
Dietary influence on tumor growth has been the subject of scientific investigations for years.1-6 Diet affects hormones and it is widely recognized that breast, ovarian and prostate cancers are hormonally driven. The reality is all cancers are hormonally driven. Hormones protect both the mother and fetus during pregnancy. Estrogens are classified as carcinogens and can cause and promote cancer in both men and women under certain conditions. Therefore it is important to recognize the relationship and interaction between estrogens, phytoestrogens, estrogen receptors, cellular immunity and cancers. Armed with this knowledge the physician can manage the disease of cancer while he treats it with chemotherapy, insulin potentiation therapy (IPT), radiation, or other therapy of choice. Hormone replacement therapy (HRT) increased both cancer occurrence and death rates.7,8 To understand this, we need to recognize that HRT increased the gene expression of the estrogen receptors-alpha (ER-a) on cells. Cancers are always involved with the ER-a receptors. The Gene Alteration chart (Figure 1) shows the gene expressions of ER-a and estrogen receptor-beta (ER-b) in healthy 20-year-old females (left side) and the gene expressions in four classes of postmenopausal women (right side).8 Postmenopausal women have increased ER-a sites (red) and decreased ER-b sites (blue) in their cells. Moving to the right on the chart, we see HRT causes increases in the ER-a receptor sites, which increases cancer risks because all cancers are involved in the ER-a receptor sites. The ER-b sites on cells play a role in immunity and killing of cancer in the cells. Moving farther to the right on the chart, it shows that soy phytoestrogens reduce the ER-a sites in both the HRT-treated postmenopausal women and the untreated alike. This reduces their cancer risks of all types because the ER-a is in all of our cells except those in the urinary tract. HRT, in addition to increasing ER-a, increased cellular levels of matrix-metalloproteinase 9120 times higher than normal (Figure 2).9 MMP is the enzyme that allows cancer cells to metastasize. The higher death rate associated with HRT is attributed to the increased MMP.10-19 Soy phytoestrogens reduce cancer risks in both HRT-treated and untreated postmenopausal women by reducing ER-a sites in cells (Figure 1).8,25Soy phytoestrogens reduce cancer metastases by reducing cellular MMP 120 times lower in HRT-treated women (Figure 2).9
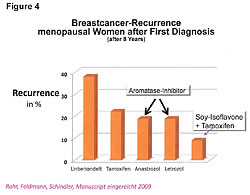
Lessons may be drawn from results of studies presented herein applicable to fermented soy benefits for both (1) nutrition to support wellness for normal populations and (2) in conjunction with health-care providers, adjuvant nutrition for people concerned with cancer. If, as stated, soy phytoestrogens reduce both cancer risks and its metastasis, is there any truth to the statement that has been made by Big Pharma, and repeated by most doctors and respectable health newsletters that (1) “Women who take Tamoxifen should not take soy products because the soy phytoestrogens occupy the same receptor site as Tamoxifen … and (2) reduce the effectiveness of the treatment, and (3) in addition, the soy phytoestrogens raise the estrogenic index and make those estrogen receptor positive cancers grow quicker”? There is no truth in any part of the three points made in the quoted statement (Figure 4).26,27
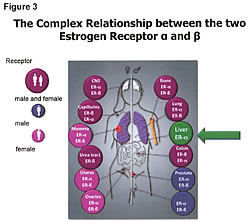
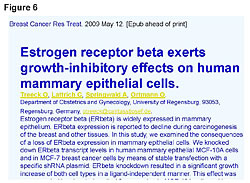
First, we need to understand that soy phytoestrogens are not estrogens, nor do they act like estrogens. There is not one study showing that phytoestrogens act like estrogens. All estrogens go to the ER-a sites and are absorbed in the liver (95%-98%), which only has ER-a site activity. There is no ER-b in the liver (Figure 3).20 Second, the soy phytoestrogens do not go to the same receptor site as Tamoxifen. Studies show that soy phytoestrogens have very low affinity for the ER-a sites and at least 20 to 30 times greater affinity for the ER-b site than the ER-a site. In addition, the soy phytoestrogens reduce the number of ER-a sites in a cell and increase the ER-b sites in cells.21-25 The reduction of ER-a sites by the consumption of whole soy, not isolates from soy like isoflavones, etc., results in reducing circulating estrogen levels in women.21-25 This will slow down the growth of ER-positive cancers, not promote their growth. The excreted estrogens are the 4-hydroxyestrogens and 16-hydroxyestrogens that damage DNA, cause and promote cancer growth. This reduction in circulating estrogens lowers the estrogenic index and is beneficial to the ER-positive cancer patients. Third, the soy phytoestrogens do not reduce the effectiveness of Tamoxifen treatments nor does it reduce the effectiveness of Big Pharma’s aromatase inhibitor products (Figure 4; Femora, Arimidex, etc.).26,27 Bette J. Caan of Kaiser Permanente in Oakland was quoted as saying that her study was interesting because “it did not counteract any of the benefits of tamoxifen and, at high levels, soy is as effective as tamoxifen.”26 Tamoxifen and soy phytoestrogens go to different receptor sites and work in different areas of the cell cycle.27 They are synergistic. The reoccurrence of ER-positive breast cancer, at 8 years’ survival time, was reduced 60% with the combination treatment of soy and Tamoxifen.26,27 The death rate was reduced with the combined treatment.27 Both Tamoxifen and aromatase inhibitors work by reducing the estrogen levels entering the ER-a sites. Soymilk consumption for two weeks lowers estradiol levels 27% in Japanese women, and they have lower circulating levels of estrogens because of their dietary soy, which is fermented.21-25 Unfermented soy products have undesirable characteristics. Fermentation improves bioavailability and eliminates undesirable compounds found in nonfermented soy. All soy products are not created equal, and results presented herein may not be achieved with unfermented or lower-quality soy products.28 The problem with understanding the antiestrogenic properties of soy originates in the common belief that phytoestrogens are estrogens. They are antiestrogens. To add more confusion, Prof. Jan Gustafsson, 1995 Nobel Prize winner and Chairman of the Nobel Prize Committee, while working at the Karolinska Institute in Sweden discovered what he named the estrogen receptor beta site (Figure 5). The ER-b site is not where estrogens go. Estrogens go to the ER-a site.29-31 Despite the confusion added by the name, the ER-b discovery opened a whole new area of our understanding on cellular immunity and the complex relationship between the ER-a and the ER-beta receptor sites. Compounds that occupy the ER-b receptor site are anti-estrogenic, regulate immunity and kill cancer in the cell (Figure 6).29-38Compounds that go to the ER-a site are carcinogenic, estrogenic, and involved with increased cancer risks.29-38 We should think of ER-b as the 3-beta adiol receptor site. Adiol is nature’s key player in our cellular immunity and it goes to the ER-b site.
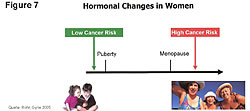
The naturally produced adiol provides the cellular immunity of men, women, children, and the developing fetus.55 Adiol governs our immunity for life. It is in us when we are born and throughout life. The immunity that it controls does not involve white blood cells, natural killer (NK) cells, T-cells, or other blood chemistry. It is not what people think about when we talk about increasing our immune system. The adiol works within the cell, not outside the cell like NK cells, T-cells, macrophages, and so on. It builds bones and governs cellular immunity. The soy phytoestrogens occupy the 3-beta adiol receptor site, which is known as ER-b, and provide the same cellular immunity protection. The problem with estrogen is not that estrogens are carcinogens. The problem is that estrogens cause cancer when a person is deficient in the number of ER-b sites on the cells – and a deficiency of the 3-beta adiol and other anticancer compounds that go to the ER-b sites.28-32,55 This is supported by the fact that we don’t see women in the prime of life getting ovarian cancers when estrogen levels are high (Figure 7).33Women get ovarian cancers when estrogens are low. Younger women have higher circulating levels of estrogens but also have greater numbers of ER-b on their cells and higher hormonal levels of adiol. The higher adiol levels in addition to more ER-beta receptors to deliver the adiol to cells protect the younger women from cancers. Men get prostate cancer in old age. Old men have higher estrogen levels than women of the same age. The estrogens increase ER-a sites on prostate cells, damage DNA, and increase cancer risks. The hormone level of adiol is reduced in both men and women with age. The reduction in adiol’s protection from estrogens and other carcinogens results in increased cancer rates. The soy phytoestrogens occupy the same receptor site as adiol and provide the same cellular immunity.29-37,55
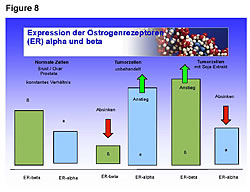
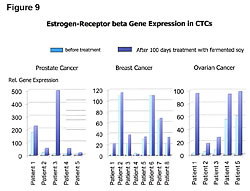
Healthy people have greater numbers of ER-b sites on their cells than ER-a (Figures 8,9).25 Cancer patients have greater numbers of ER-a sites on their cells than ER-b sites (Figure 8).25 Fermented soy increases ER-b sites on circulating tumor cells in breast, ovarian, and prostate cancers (Figures 8,9).25 In addition, it decreases the number of ER-a sites on circulating breast, ovarian, and prostate tumor cells (Figures 8,9).25 The first two columns in Figure 8 compare the ER-b to the ER-a in breast, ovarian, and prostate cells of healthy people. The two columns in the middle of Figure 8 shows the number of ER-a and ER-b on breast, ovarian and prostate cancer cells. The third set of columns on the right of Figure 8 shows the restoration to a healthy ratio of ER-a and ER-b on breast, ovarian, and prostate cancer cells by fermented soy.
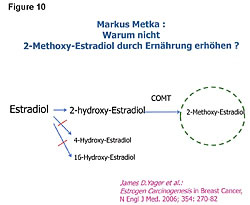
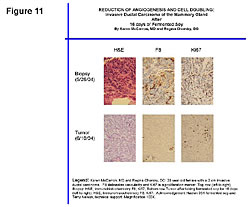
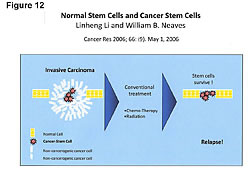
The goal of appropriate cancer prevention and treatment of cancer should be to increase both the number of ER-b sites and the anticancer compounds that have an affinity to the ER-b receptor sites – and at the same time reduce the number of ER-a receptor sites on the cells. One of the most potent anticancer compounds produced in the body is 2-methoxyestradiol (Figure 10).34,36”‘39 This compound kills pancreatic, liver, and all cancer cells. How? It goes to the ER-b receptor sites. The key is to have plenty of ER-b sites so that greater amounts of the anticancer compounds can be delivered to the cells. Remember, cancer patients have low numbers of ER-b sites. The ER-b site kills the cancer cell from within. There are no adverse side effects because this killing is within the cancer cell; it is nontoxic. Therefore, as stated earlier, the proper treatment of cancer should include management of the estrogens, ER sites, estrogen metabolism, and cellular immunity. The benefits from managing estrogen metabolism to both a healthy person and a cancer patient depend on the method employed to improve the ratio of the “good” and “bad” estrogens. Indole-3-carbinol (IC3), diindolylmethane (DIM), and soy are the three dietary compounds that improve the ratios between the 2-, 4-, and 16-hydroxyestradiol. The choice among IC3, DIM, and soy is a matter of consumer choice. However, in addition to improving estrogen metabolism, fermented soy has been shown to increase the P-53 tumor suppressor gene expression, and exhibit significant anticancer and antiangiogenic influences.25,27,28,41,43,44,54-56 In addition, fermented soy increases BRCA1 and BRCA2 gene expressions and reduces HER2 gene expression.25,43 BRCA1 and BRCA2 proteins repairs DNA damage in breast cells. Women with low levels of BRCA1 and BRCA2 gene expressions have increased rates of breast cancer. Reduced HER2 gene expression slows breast cancer grow rates. Twenty four percent of women are reported to be HER2 positive. The drug Herceptin reduces HER2 gene expression, but does not cross the blood-brain barrier, whereas fermented soy does. Figure 11 shows the antiangiogenic effects of fermented soy on a breast tumor after 16 days.44 The top left slide shows the blood feeding the tumor at the beginning of the time period. The comparative photos on the bottom show the blood supply shut-off that feeds the tumor and improvements in other cellular DNA markers in the tumor. Fermented soy allows the physician to manage cachexia, anorexia, malnutrition, protein calorie malnutrition, and the toxic side effects of chemotherapy treatments, in addition to improvements in estrogen levels, receptors (both ER-a and ER-b), estrogen metabolism, and cellular immunity.25,44,55 Clinical studies show fermented soy does not reduce the effectiveness of chemotherapy treatments.45,56,57 Fermented soy increases the effectiveness of chemotherapy, shuts off the mutation pathway of cancer cells, reduces multidrug resistance, and reduces the toxic side effects of chemotherapy and radiation treatments. Karmanos Cancer Center, Wayne State University (Detroit, Michigan), has reported that “all cancer cells try to mutate within two hours of receiving chemotherapy treatments. The mutation pathway of the cancer cell is nuclear factor kappa beta (NF-Kb), and the soy phytoestrogens shut off that mutation pathway and cancer cell kill is enhanced by both chemotherapy and radiation therapies.56 The management of estrogens, estrogen receptors, estrogen metabolism, and cellular immunity becomes more important in cancer treatment now that we know more about the cell subsets in tumors. We know only 1/10 of 1% and not more than 9/10 of 1% of the cells in a tumor are cancer stem cells. These are the cells in the tumor that can metastasize to vital organs. These are the ones that kill the cancer patient. We now also know that chemotherapy and radiation do not kill cancer stem cells (Figure 12).27,39,46,47,49,55 Chemotherapy and radiation only kill the cells in a tumor that are not dangerous unless tumor size is a factor due to space limitations, as in brain tumors. This is why the death rate has not changed. The war on cancer goes on because chemotherapy and radiation do not kill the cancer stem cells.27,39,46,47,49,55,56
Chemotherapy, in addition to lacking the ability to kill cancer stem cells, cause cell mutations that are resistant to subsequent chemotherapy treatments. Doxorubicin treatment of breast cancer reduces the antiapoptosis gene expression BCl2 but also decreases the proapoptosis gene expresson BAX, whereas fermented soy reduces BCL2 and increases BAX.25,57 It has been found that the ratio of BAX:BCL2, rather than BCL2 alone, is important for the survival of drug-induced apoptosis in leukemia cell lines.57,58 Reduced levels in BCL2 in all cancer cells tested after treatment with soy isoflavones is reduced.56 After chemotherapy treatment, the growth of the surviving cancer stem cells is faster because there is a greater blood supply to feed the surviving cancer stem cells. The cancer stem cell growth was restricted when they were less than 1% of the total cells in the primary tumor. When the tumor shrinks, the blood supply to feed the surviving cancer stem cells is increased. A major obstacle to their growth is now removed. The cancer patient is now destined to greater numbers of cancer stem cells and mutated cells in the tumor. Both are resistant to the treatments that were hoped to cure the patient of their cancer. What can possibly kill the cancer stem cells? Chemotherapy and radiation do not kill cancer stem cells. Tamoxifen and aromatase inhibitors both work by reducing estrogens going to the ER-a sites. Both are poor candidates for the job at hand because cancer stem cells do not have ER-a sites on them. Therefore, neither Tamoxifen or aromatase inhibitors would be expected to kill cancer stem cells. Our focus should turns to the body’s cellular immunity system: it kills cancer stem cells.55The adiol and compounds that have an affinity to the adiol receptor site (ER-b) provides cellular immunity and protection from cancer stem cells in men, women, children, and the fetus.32-39,51-55 It protects both the mother and her fetus during and after pregnancy.55 How does this work?
The cancer cells in the mother are killed by the same immune-system regulating hormones that work through the ER-b sites. They kill cancer cells in the mother and protect the fetus from being killed by the mother’s immune system.54,55 In summary, the management of estrogens, estrogen receptors, estrogen metabolism, and cellular immunity can result in decreased ER-a, increases the ER-b, increased levels of the immune-stimulating, anticancer compounds that have affinity to the ER-b site, reduced circulating levels of the DNA-damaging, cancer-promoting estrogens, and delivery of increased quantities of anticancer compounds to the cellular immune-regulating ER-b sites. These are natural mechanisms that our bodies use to provide cellular immunity and kill cancer, including stem cells. The professional health-care worker can help patients restore their natural cellular immunity in cases where their system has become imbalanced. Fermented soy occupies the 3-beta adiol receptor site, which we know as ER-b, and provides the same protection.55 The problem with soy’s acceptance has been due to misunderstanding the nature of phytoestrogens. The information in this article should help to counter the myth that soy phytoestrogens are estrogenic and promote cancer when in fact they are beneficial antiestrogenic compounds. They are extremely beneficial to the ER-positive cancer patients as well as healthy people. Hopefully, the clinical importance of fermented soy in the management of estrogens, estrogen receptors, estrogen metabolism, and cellular immunity to promote health, as presented herein, will help the professional health-care worker in achieving the goal of longer, healthier, lives for their patients.
Walter H. Wainright is the founder and president of Haelan Research Foundation, a US government approved 501(c)(3) public foundation. Mr. Wainright, an honors graduate of Tulane University, is an internationally known lecturer with 20 years’ experience in researching the prevention and treatment of cancer and other chronic diseases with soy phytochemicals. Mr. Wainright is collaborating with the BioFocus Institute in Recklinghausen, Germany, on reversing DNA damage in cancer cells with soy phytochemicals.
View the print-quality graphics in a 2.89MB .pdf Notes |
||||||||||||||

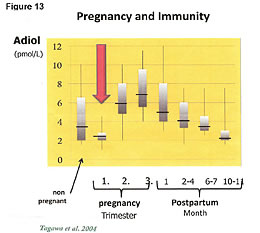 The protection of the mother and child by hormones during pregnancy is complex but a short version of the hormonal mechanisms and its actions are as follows: The chart in Figure 13 tells the story.54 In order for a woman to have a surviving baby, her immunity must be lowered. Every month, her body lowers the naturally produced adiol compound that controls her cellular immunity. If the adiol and its associated higher levels of immunity were not reduced, the fetus would be killed by her immune system. During the monthly menstrual cycle, there is a period of reduced immunity (lowered adiol) and increased inflammation. The inflammatory cytokines reduce the structural integrity of the membranes in the uterus, which has plenty of stem cells, and the capillaries bleed. This produces the blood supply we know as the menstrual cycle. This blood supply will feed the embryo if the women becomes pregnant. The monthly menstrual blood serves the purpose of feeding the fetus prior to formation of the umbilical cord. The umbilical cord is formed in the second and third trimester of pregnancy. The immunity to the fetus is provided by compounds in the umbilical cord that are precursors to the production of adiol. Adiol builds bones in the baby during growth and regulates immunity. Once the umbilical cord has grown (2nd month), the woman’s body upregulates the production of adiol. This increased cellular immunity kills cancers in the mother that grow during her lowered immunity that allows her fetus to survive.54,55
The protection of the mother and child by hormones during pregnancy is complex but a short version of the hormonal mechanisms and its actions are as follows: The chart in Figure 13 tells the story.54 In order for a woman to have a surviving baby, her immunity must be lowered. Every month, her body lowers the naturally produced adiol compound that controls her cellular immunity. If the adiol and its associated higher levels of immunity were not reduced, the fetus would be killed by her immune system. During the monthly menstrual cycle, there is a period of reduced immunity (lowered adiol) and increased inflammation. The inflammatory cytokines reduce the structural integrity of the membranes in the uterus, which has plenty of stem cells, and the capillaries bleed. This produces the blood supply we know as the menstrual cycle. This blood supply will feed the embryo if the women becomes pregnant. The monthly menstrual blood serves the purpose of feeding the fetus prior to formation of the umbilical cord. The umbilical cord is formed in the second and third trimester of pregnancy. The immunity to the fetus is provided by compounds in the umbilical cord that are precursors to the production of adiol. Adiol builds bones in the baby during growth and regulates immunity. Once the umbilical cord has grown (2nd month), the woman’s body upregulates the production of adiol. This increased cellular immunity kills cancers in the mother that grow during her lowered immunity that allows her fetus to survive.54,55
Pingback: WeeksMD » Soy is helpful for cancer. (hint: ERbeta)